1
This guideline is designed
to help establishments
determine:
• The supporting
documentation needed
when submitting labels
that bear an animal
raising claim.
• Whether a modified
label with an animal
raising claim that has
been approved is
required to come back
for approval or can be
changed generically.
• How to add additional
suppliers to a label with
an animal raising claim
that has been approved.
This guideline is designed
to help establishments
determine:
• The supporting
documentation needed
when submitting labels
that bear an animal
raising claim.
• How to add additional
suppliers to a label with
an animal raising claim
that has been approved.
Food Safety and Inspection Service
Labeling Guideline on
Documentation Needed to Substantiate Animal
Raising Claims for Label Submissions
December 2019

2
FSIS Compliance Guideline for Label Approval
Table of Contents
Preface ............................................................................................................................ 3
Documentation Needed to Substantiate Animal Raising Claims for Label Submission ... 6
Animal Welfare and Environmental Stewardship……………………………...…… 7
Breed……………………………………………………………………………………. 8
Diet………………………………………………………………………………….…… 9
Living/Raising/Raising Conditions……………………………………………….…. 10
Negative Antibiotics Use - Livestock/Red Meat……………....……………………12
Negative Antibiotics Use - Poultry……………………...…..…………………….. ..12
Negative Hormones Use………………………………………………………….…. 13
Source/Traceability…………………………………………………………………... 14
Third-Party Certification……………………………………………………………... 15
Organic……………………………………………………………………………..…. 15
Adding a New Supplier………………...…………………………………………….………. 16
Label Example……...………………………………………………………………………… 17
Additional Resources .................................................................................................... 18

3
Preface
What is the purpose of this Compliance Guideline?
The purpose of this compliance guideline is to
outline the documentation that establishments
need to submit in support of label applications
for products that bear animal raising claims.
The Food Safety and Inspection Service (FSIS)
is the Agency in USDA with the responsibility for
ensuring that the labeling of meat and poultry
products is truthful and not misleading. Labeling
bearing claims referring to the way that the
source animal for a meat or poultry product was
raised need to be evaluated and approved by
FSIS prior to use.
For the past 25 years, FSIS has evaluated
animal raising claims by considering information
on animal raising practices submitted by
companies as part of their label approval
requests. The Agency has approved such claims if the animal raising information
submitted with the label application supported the claim being made and the claim is
truthful and not misleading.
FSIS developed this guideline to respond to numerous requests to the Labeling
Program and Delivery Staff (LPDS) through phone calls, askFSIS questions, and other
correspondence regarding the type of information needed to support the approval of
labels bearing animal raising claims. This guideline is intended to facilitate the approval
process for labels bearing animal raising claims. The information in this guideline is
provided as guidance to assist meat and poultry establishments and is not legally
binding from a regulatory perspective.
Who is this guideline designed for?
This guideline is for establishments that are designing or modifying meat or poultry
product labels with animal raising claims. The establishment must determine what
supporting documentation is required for the various types of animal raising claims.
This guideline will assist the establishment in making this determination.
How will FSIS verify whether establishments meet requirements related
to this guideline?
FSIS in-plant personnel verify that establishments comply with labeling regulations,
when performing the General Labeling task assigned through the Public Health
Information System (PHIS). For product bearing animal raising claims, in-plant
personnel verify whether establishments maintain an FSIS approved label on file.
Animal raising claims are special statements and claims that establishments are
Key Points
The following are examples of
animal raising claims that are
required to be approved by FSIS
prior to use in commerce:
1. Raised Without Antibiotics
2. Organic
3. Grass Fed
4. Raised Without the Use of
Hormones

4
required to submit to FSIS for approval for compliance with 9 CFR 412.1, USDA’s Label
Approval Regulations.
Changes made to the guideline from the previous version
After reviewing the comments received, FSIS has revised the guideline by section as
follows:
• Product Labeling: Use of Animal-Raising Claims on the Labels of Meat or Poultry
Products
o Added information about labeling needed for products bearing claims certified
by third-party organization, including when products certified as “organic”
need to disclose the certifying entity’s website address on the product label.
o Added information about carrying claims forward on additional products.
o Removed age claims section because establishments are not using these
claims.
o Animal Welfare and Environmental Stewardship Claims:
o Added descriptive language or information (terminology) that should
accompany these claims to explain the meaning of the claim to
consumers, including the type of information that needs to appear on
the label when the product is certified by a third-party organization.
o Breed claims:
o Added information about carrying these claims forward to other
products.
o Living- or Raising-Condition Claims:
o Reorganized section for clarity regarding labeling terminology and
recommended documentation for approval.
o Added information about additional terminology that typically
accompanies these claims to explain the meaning of the claim to
consumers, including where the information must appear on the label.
o Added information on the use of “Free Range” and synonymous claims
(“Free Roaming,” “Pasture Fed,” Pasture Grown,” “Pasture Raised,”
and “Meadow Raised”) on labels of poultry products and the
documentation needed to substantiate these claims.
o Raised Without Antibiotics – Livestock/Red Meat or Poultry:
o Added “Raised Antibiotic Free” and “No added antibiotics” as examples
of claims that may be used to disclose the fact that animals were not
administered antibiotics at any point in the animal production process.
o Added information on claims that include the term “sub-therapeutic
antibiotics” to ensure that consumers understand that the claim means
that antibiotics may only be administered in the event of an illness and
includes the circumstances for which FSIS will approve labels bearing
these claims.
o Raised Without Hormones (No Hormones Administered or No Steroids
Administered):
o Updated information to clarify that FSIS will no longer require a
qualifying statement on pork products labeled with “raised without
hormones” claim because Federal law permits the use of certain
hormones in swine, e.g., for gestation.
5
o Added new examples of this type of claim.
o Updated information to clarify why a qualifying statement is necessary
for products made from a kind or species for which Federal law
prohibits hormone use and to emphasize that this information must be
displayed on the label in a manner that is likely to be read and
understood by the ordinary individual for FSIS to approve the claim..
o Third-Party Certification:
o Added information about documentation needed to support labels
bearing animal raising claims that have been “Verified” or “Certified” by
third-party organizations.
o Added information about “organic” claims, including other claims that
could be substantiated with an Organic Certificate.
o Added section on procedures for adding an additional supplier for a label with
animal-raising claims that was previously approved by FSIS.
The updated guideline is posted at:
https://www.fsis.usda.gov/wps/portal/fsis/topics/regulatory-compliance/compliance-
guides-index/. FSIS will update this document, as necessary.
What if I still have questions after I read this guideline?
If the desired information cannot be found within the Compliance Guideline, FSIS
recommends that users search the publicly posted Questions & Answers (Q&As) in the
askFSIS database or submit questions through askFSIS. Documenting the questions
helps FSIS improve and refine present and future versions of the Compliance Guideline
and associated issuances.
When submitting a question, use the Submit a Question tab, and enter the following
information in the fields provided:
SubjectField: “Documentation Needed to Substantiate Animal Raising Claims”
QuestionField: Enter question with as much detail as possible.
ProductField: Select Labeling from the drop-down menu.
CategoryField: Select Labeling Regulations, Policies and Claims from the drop-
down menu.
Policy Arena: Select Domestic (U.S.) only from the drop-down menu.
When all fields are complete, press Continue.
Congressional Review Act
Pursuant to the Congressional Review Act at 5 U.S.C. 801 et seq., the Office of
Information and Regulatory Affairs has determined that this guideline is not a “major
rule,” as defined by 5 U.S.C. 804(2).
6
Product Labeling: Use of the Animal Raising Claims on the Labels of Meat and
Poultry Products
A Federal establishment is required to use labels that are in compliance with the
Federal Meat Inspection Act (FMIA; 21 U.S.C. § 601, 607) and the Poultry Products
Inspection Act (PPIA; 21 U.S.C. § 451, 457) (the Acts), and the implementing
regulations. Requirements include all mandatory labeling requirements as prescribed in
Title 9 of the Code of Federal Regulations (CFR) section 317.2 and Part 381 Subpart N.
Although FSIS does not exercise its authority of prior label approval to point of purchase
materials (e.g., pamphlets and placards) displayed in conjunction with products sold at
retail and bearing animal raising claims, FSIS does require these materials be neither
false or misleading, in compliance with the Acts and Federal regulations.
As stated in 9 CFR 412.1, labels with special statements and claims are required to be
approved by FSIS prior to use in commerce.
Labels bearing animal raising claims are required to be submitted to LPDS with specific
documentation to support all animal raising claims that appear on that label. Examples
of animal raising claims include, but are not limited to: Raised Without Antibiotics, Grass
Fed, Free-Range, and Raised Without the Use of Hormones. For most animal raising
claims, the documentation typically needed to support these claims is:
1. A detailed written description explaining the controls used for ensuring that the
raising claim is valid from birth to harvest or the period of raising being
referenced by the claim;
2. A signed and dated document describing how the animals are raised which may
include feed formulations (e.g., vegetarian fed, raised without antibiotics, grass
fed), to support that the specific claim made is truthful and not misleading;
3. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution;
4. A written description for the identification, control, and segregation of non-
conforming animals/product; and
5. If a third-party certifies a claim, a current copy of the certificate from the certifying
organization.
NOTE: If the claim was certified by a third-party certifying organization, FSIS will not
approve the label bearing the claim if it does not include the certifying entities name,
website address,
1
and logo, when the organization has a logo. An asterisk or other
symbol must connect the claim to this information.
1
Products certified as “organic” would not need to disclose a website address on the label, except when the
address is required under 7 CFR Part 205.

7
In general, a purchased product bearing an approved animal raising claim may be used
to support the claim in lieu of numbers 1 – 3 above. If a company purchases product
bearing animal raising claims and would like to carry forward those claims onto its
labeled product, the company needs to provide a copy of the purchased product label
and segregation procedures when entering their federal establishment. However,
companies cannot carry forward certified claims, logos and/or websites from purchased
products that are certified by a third-party entity unless the companies that are carrying
the claim forward are also under that same certification. Companies cannot carry
forward USDA organic claims, logos and/or websites without being certified organic
themselves.
Types of Animal Raising Claims and Guideline on the Documentation Needed to
Substantiate the Claims
Animal Welfare and Environmental Stewardship
These claims describe how animals are raised based on the care they receive by the
producer or how the producer maintains the land and replenishes the environment.
FSIS has not defined these claims in regulations or policy guidelines. For animal welfare
claims, such as “Raised with Care,” or environmental stewardship claims, such as
“Sustainably Raised,” FSIS will only approve a claim if a statement is provided on the
label showing the name of the entity that established the standard and includes
additional terminology explaining the meaning of the claim for consumers, e.g., "TMB
Ranch Defines Raised with Care/Sustainably Raised as [explain the meaning of the
claim on the label]." If the entity has a website that describes the standards used to
define the claim, the label may provide the website address instead of explaining what
the claim means on the product label, e.g., “Raised with Care as defined by TMB Ranch
at: [website address].” As an alternative, animal welfare and environmental
stewardship claims can be certified by a third-party certifying organization that posts the
standards used to define the claim on its website. If the claim is certified by a third-party
certifying organization, FSIS will not approve the label bearing the claim if it does not
include the certifying entities name, website address, and logo, when the organization
has a logo.
The claims may appear on any panel of the package. The additional terminology that
explains the meaning of the claim may appear with the claim or may be connected to
the claim by an asterisk or another symbol and placed elsewhere on the same panel
that bears the claim. For example, if a claim is made on the principal display panel
(PDP), the part of the label most likely to be seen by consumers when offered for retail
sale, the explanation of the claim’s meaning may be placed with the claim or placed
elsewhere on the PDP provided the claim and explanation are connected by a symbol.
If the claim is certified by a third-party certifying organization, an asterisk or other
symbol must connect the claim to the certifying entities name, website address, and/or
logo, when the organization has a logo, for FSIS to be able to approve the label.
Examples of these types of claims include, but are not limited to: Humanely Raised*,
Sustainably Farmed*, and Raised with Environmental Stewardship*.
8
*TMB Ranch defines “Humanely Raised”/”Sustainably Farmed/Raised with
Environmental Stewardship” as [insert description of standards used to define the
claim]
Documentation needed:
1. A detailed written description explaining the meaning of the animal welfare or
environmental stewardship claim and the controls used for ensuring that the
raising claim is valid from birth to harvest; or the period of raising being
referenced by the claim;
2. A signed and dated document describing how the animals are raised to support
that the claims are not false or misleading;
3. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution; and
4. A written description for the identification, control, and segregation of non-
conforming animals/product (e.g., how animals not raised in accordance with the
specific animal welfare guidelines or stewardship program are segregated from
animals eligible to bear the claim).
Breed
Breed claims refer to the declaration of a specific breed of livestock or poultry.
Examples of this type of claim include, but are not limited to: Angus, Wagyu (American
Kobe), Hereford, Berkshire, Duroc, Muscovy, Silkie, and heritage poultry, pork or beef
breeds.
Documentation needed:
1. A signed and dated document that substantiates the breed claim, e.g., under a
Agricultural Marketing Service (AMS) Certified Meat and Poultry Program or a
certificate from a breed organization;
2. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution;
3. Documentation to support the breed by phenotype (for example, hide color) or
genotype (traceable to one registered parent or two registered grandparents with
a breed association); and
4. A written description for the identification, control, and segregation of non-
conforming animals/product.
Alternatively, a purchased product label may be used in lieu of numbers 1 – 3 above. If
a company purchases product bearing a breed claim and would like to carry forward the
claim on to their product, the company needs to provide a copy of the purchased label
and segregation procedures for product entering the federal establishment when
submitting for label approval.
NOTE: See label example

9
Diet
Diet claims refer to what animals are fed prior to harvest and processing. These claims
require that the animals only eat the diet claimed for the lifetime of the animal, with the
exception of milk consumed prior to weaning.
FSIS considers Grassfed, Grass Fed and Grass-Fed synonymous terms. “Grass Fed”
or “100% Grass Fed” claims may only be applied to meat and meat product labels
derived from cattle that were only (100%) fed grass (forage) after being weaned from
their mother’s milk. The diet must be derived solely from forage, and animals cannot be
fed grain or grain by-products and must have continuous access to pasture during the
growing season until slaughter. This means 100% grass-fed animals are never confined
to a feedlot. Forage consists of grass (annual and perennial), forbs (e.g., legumes,
Brassica), browse, or cereal grain crops in the vegetative (pre-grain) state. Hay,
haylage, baleage, silage, crop residue without grain, and other roughage sources may
also be included as acceptable feed sources. Routine mineral and vitamin
supplementation may also be included in the feeding regimen. If incidental
supplementation occurs due to inadvertent exposure to non-forage feedstuffs or to
ensure the animal’s wellbeing at all times during adverse environmental or physical
conditions, the producer should provide a signed and dated document to the
establishment attesting the above incident is not a routine occurrence. The
establishment should include this information as part of the labeling documentation
verifying the product qualifies for a grass fed claim.
When animals have less than 100-percent access to grass or forage the partial “grass
fed” claim must accurately reflect the circumstances of raising, e.g., “Made from cows
fed 85% grass and 15% corn.”
The claim “Grass Finished” is not the same as “Grass Fed” because animals that are
“grass finished” can be fed grain, in which case the claim “Grain Fed, Grass Finished”
would be truthful and not misleading.
Historically, the AMS Grass (Forage) Fed Marketing Claim Standard was considered
one form of proof to FSIS that the claim “grass fed” was truthful and not misleading. In
January 2016, AMS withdrew the standard. This change did not change FSIS’s
documentation requirements for companies wishing to label their products as “Grass
Fed.”
Examples of this type of claim include, but are not limited to: Grass (Forage) Fed, Grain
Fed, Vegetarian Feed, Raised Using Vegetarian Feeds [This means all vegetable feeds
and no animal products (e.g., whey) are fed to the animal.], Raised Using Vegetarian
Feeds (with a disclaimer to clarify animal products are fed to the animal for a certain
period of time, e.g., “except for dairy products fed from birth to eight weeks” or “after 8
weeks”), and Fed No Animal By-Products.
Documentation needed:
1. A detailed written description explaining controls for ensuring that the raising
claim is valid from birth to harvest or the period of raising being referenced by the
10
claim; (e.g., controls to ensure cattle that are supposed to be raised 100% grass
fed are not fed grains);
2. A signed and dated document describing the diet of the animals to support that
the claims are not false or misleading;
3. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution; and
4. A written description for the identification, control, and segregation of non-
conforming animals/product.
As an alternative, a producer may use the USDA Process Verified Program (PVP)
(carried out by AMS) to verify their product meets their own grass-fed standard in lieu of
documentation needed for 1-4 above. The USDA PVP is a voluntary, user-fee
verification service that offers applicants a unique way to market their products to
customers using clearly defined, implemented, and transparent process points. An
applicant’s program may include one or more agricultural processes or portions of
processes independently verified by a qualified AMS auditor. Examples of process
points include, but are not limited to: adherence to a recognized standard or one
created by a company or organization; definitions included within this guidance; a
production, raising and/or handling practice that provides specific information to
consumers to enable them to make informed decisions on the products that they buy; or
a characteristic, practice, or requirement that is specifically requested by a customer or
consumer. AMS auditors conduct a comprehensive review of a company’s program,
which includes an on-site audit of all facilities and phases of the operation that impact
process verified points. Additional information about the USDA PVP service, shield,
certificate, and official listing are available at
www.ams.usda.gov/services/auditing/process-verified-programs.
There are a number of private third-party certification programs that accomplish the
same objectives. If the claim is certified by a third-party organization, an asterisk or
other symbol must connect the claim to the certifying entities name, website address,
and/or logo, when the organization has a logo.
NOTE: See label example
Living/Raising/Raising Conditions
These claims refer to the environment in which the animals or birds were raised during
their lifespan.
Examples of this type of claim include but are not limited to: Cage or Crate Free, Free
Range**, Not Confined, Free Roaming, Pasture Fed, Pasture Grown, Meadow Raised,
and Pasture Raised.
NOTE: For all of the above claims, additional terminology is necessary on the label to
define its meaning on livestock products and to convey that the animals were never
confined to a feedlot.
Because FSIS has not defined these claims in the regulations or policy guidelines,
nearly all living/raising conditions claims need to describe the standards used to define

11
that claim as applied to that particular product, e.g., “Cage free. Chickens were never
confined to cages during raising.” The information must appear with the claim or be
connected by a symbol on the same panel on which the claim appears. As an
alternative, these living/raising claims can be certified by a third-party certifying
organization that posts its standards for defining the claim on its website. If the claim is
certified by a third-party certifying organization, FSIS will not approve the label bearing
the claim if it does not include the certifying entity’s name, website address, and logo,
when the organization has a logo. An asterisk or other symbol must connect the claim to
this information.
**Based on consultations with AMS, FSIS determined that additional terminology is not
needed on the label for the claim Free Range or synonymous claims (“Free Roaming,”
“Pasture Fed,” Pasture Grown,” “Pasture Raised,” and “Meadow Raised”) on poultry
products. However, for FSIS to approve these claims, additional documentation must be
submitted to substantiate the claim. Specific details about what additional information is
needed is provided below.
Documentation needed:
1. A detailed written description explaining controls for ensuring that the animals are
raised in a manner consistent with the meaning of the raising claim that is valid
from birth to harvest or the period of raising being referenced by the claim.
2. A signed and dated document describing how the animals are raised to support
that the claims are not false or misleading;
3. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution; and
4. A written description of the identification, control, and segregation of non-
conforming animals/product.
As part of 1 or 2 above, for the claim Free Range on poultry products, the
documentation must describe the housing conditions for the birds and demonstrate
continuous, free access to the outside throughout their normal growing cycle. During the
winter months in a northern climate, birds are not free range if they stay in poultry
housing or coops all winter. Producer documentation to support the use of the claim for
birds raised in a northern climate during winter months would also need to describe the
housing conditions for the birds and demonstrate continuous, free access to the outside
throughout their normal growing cycle.
As part of 1 or 2 above, for the claims Free Roaming, Pasture Fed, Pasture Grown,
Pasture Raised, and Meadow Raised on meat or poultry products, documentation that
will typically substantiate these claims will show that the animals or birds have
continuous, free access to the outdoors throughout their usual grow-out period. For
ruminants, this means the entire grazing season for the geographical area.
NOTE: See label example
12
Negative Antibiotics Use – Livestock/Red Meat
Raised Without Antibiotics:
To use this claim, source animals cannot be administered antibiotics in their feed, water
or by injections at any point in the production process. This includes ionophores which
are recognized as antibiotics by FSIS.
Examples of this type of claim include, but are not limited to: Raised Without Antibiotics,
No Antibiotics Administered, No Added Antibiotics, No Antibiotics Ever and Raised
Antibiotic Free.
No Sub-Therapeutic Antibiotics:
FSIS will approve a claim that states that animals have not been administered sub-
therapeutic antibiotics if the claim is part of a complete claim that explain what the term
“sub-therapeutic” means, e.g., “No sub-therapeutic antibiotics. Animals do not receive
antibiotics on a daily basis; animals only receive antibiotics in the case of illness.”
Other examples of this claim that FSIS is likely to find to be truthful and not misleading
include: “Beef Raised with No Sub-Therapeutic Antibiotics Ever, animals may be given
antibiotics for the treatment of illness” or “Beef Raised with No Sub-Therapeutic
Antibiotics, Animal do not receive antibiotics on a daily basis only in the case of illness.”
Documentation needed:
1. A detailed written description explaining controls for ensuring that the animals are
not given antibiotics from birth to harvest or the period of raising being referenced
by the claim including feed formulation;
2. A signed and dated document describing how the animals are raised to support
that the claims are not false or misleading;
3. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution; and
4. A written description for the identification, control, and segregation of non-
conforming animals/product (e.g., if beef raised without the use of antibiotics
need to be treated with antibiotics due to illness).
NOTE: See label example
Negative Antibiotics Use – Poultry
Raised Without Antibiotics
For FSIS to find this claim to be truthful and not misleading, source animals cannot be
administered antibiotics in their feed, water, or by injections. Animals cannot be
administered Ionophores, which are recognized as antibiotics by FSIS.
Examples of this type of claim include: Raised Without Antibiotics, No Antibiotics
Administered, No Added Antibiotics, No Antibiotics Ever, and Antibiotics Free.
No Sub-Therapeutic Antibiotics:

13
This claim requires additional explanation on the label to ensure consumers understand
antibiotics will be administered to the animals in the event of illness. Examples of this
claim include: “Turkey Raised with No Sub-Therapeutic Antibiotics Ever, birds may be
given antibiotics for the treatment of illness” or “Chicken Raised with No Sub-
Therapeutic Antibiotics, birds do not receive antibiotics on a daily basis only in the case
of illness.”
Documentation needed:
1. A detailed written description explaining controls for ensuring that the raising
claim is valid from birth to harvest; or the period of raising being referenced by
the claim;
2. A signed and dated document describing how the animals are raised without
antibiotics to support that the claims are not false or misleading;
3. A written description of product tracing and segregation mechanism from time of
slaughter or further processing through packaging and wholesale or retail
distribution; and
4. A written description for the identification, control, and segregation of non-
conforming animals/product (e.g., if chicken raised without the use of antibiotics
need to be treated with antibiotics due to illness).
In addition to the documentation listed above, the establishment needs to submit a
company letter (signed and on company letterhead) answering the following questions:
1. Do you use antibiotics pre-hatch in any way with respect to the eggs that you
hatch for the poultry that will bear the claim? If so, please describe how you use
antibiotics?
2. Do you inject any vaccines in ovo? If so, please state whether any of the
vaccines includes an antibiotic. If any of them does, please state what antibiotics
are used, what the antibiotics are used for, and in what amount they are used.
3. Do you inject any antibiotics in ovo? If so, please state what antibiotics are used,
what the antibiotics are used for, and in what amount they are used. What is the
withdrawal time for the antibiotics?
4. Have you verified that the poultry that you use to produce your products was not
derived from eggs or poultry that were injected or otherwise treated in any way
with antibiotics? If so, how have you verified these conclusions?
NOTE: See label example
Negative Hormones Use
Under Federal law, hormones are only approved for use in beef cattle, swine***, and
lamb production. There are no hormones approved for use in the production of poultry,
goat, veal calves, mature sheep, or exotic, non-amenable species (such as bison,
buffalo, elk, and venison). Thus, additional terminology is necessary on these labels to
convey that Federal law prohibits hormone use in these species.

14
FSIS will only approve a negative hormone claim on products made from a kind or
species for which Federal law prohibits hormone use when it is accompanied by the
qualifying statement: “There are no hormones approved for use in (kind or species
[poultry, goat, veal, mature sheep, or exotic, non-amenable]) by Federal Regulations.”
The qualifying statement must be prominently and conspicuously displayed on the label,
e.g., it appears adjacent to the claim or is in type at least one-third the height, in
accordance with 9 CFR 317.2(b) for meat products or 9 CFR 381.116(b) for poultry
products. As for any labeling claim, FSIS confirms compliance with these regulations
during the label approval process.
***NOTE: In the previous draft of this guideline, FSIS stated that no hormones had
been approved for use in the production of swine. After additional research, FSIS has
found that there are several hormones approved by the Food and Drug Administration
and marketed by drug makers to be used in swine in the United States for various
reasons (e.g., gestation). Establishments do not need to resubmit their labels for
approval to remove the previously required disclaimer statement from pork product
labels. The change can be made generically under 9 CFR 412.1.
Examples of this type of claim include: Raised Without Added Hormones, No Added
Hormones Administered, Raised Without Steroids.
Documentation needed:
1. A detailed written description explaining controls for ensuring the animals are
raised without hormones or steroids to support the claim is valid from birth to
harvest; or the period of raising being referenced by the claim;
2. A signed and dated document describing how the animals are raised (e.g.,
without the use of hormones) to support that the claims are not false or
misleading;
3. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution; and
4. A written description for the identification, control, and segregation of non-
conforming animals/product.
NOTE: See label example
Source/Traceability
This type of claim demonstrates how the animal can be traced back to its farm of origin
from birth to slaughter/harvest.
Examples of this type of claim include: Source Verified and Traceable to [Name of Farm
of Origin].
Documentation needed:
1. A detailed written description explaining controls for ensuring the source of the
animal can be verified from birth to harvest or the period of raising being
referenced by the claim;
2. A signed and dated document describing how the animals are raised to support
that the claims are not false or misleading;
15
3. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution; and
4. A written description for the identification, control, and segregation of non-
conforming animals/product; and
5. Live animal raising records demonstrating how individual animals or a group of
animals are identifiable and traceable to their farm or ranch of birth, and if
verified, the individual or entity verifying the claim.
NOTE: See label example
Third-Party Certification
Generally, FSIS accepts animal raising claims verified by a third-party auditing or
certifying program. The standards for acceptance of the third-party certifier need to be
credible and reliable. FSIS evaluates certifiers’ acceptance standards as necessary to
assess suitability for animal raising claims on labels. The label bearing the claim needs
to include the certifying entity’s name, website address, and logo, when the organization
has a logo. An asterisk or other symbol must connect the claim to this information.
Examples of this type of claim include but are not limited to: USDA PVP (administered
by AMS), Animal Welfare Approved (AWA), or GAP Step ratings (Global Animal
Partnership (GAP)).
Documentation needed:
1. A current copy of the certificate; and
2. A written description of the product tracing and segregation mechanism from time
of slaughter or further processing through packaging and wholesale or retail
distribution.
NOTE: If used in conjunction with any other animal raising claim(s) that are not covered
by the third-party organization certification, refer to the documentation needed for the
particular claim(s). However, third-party certification cannot be carried over to other
products unless the company has the same certification.
NOTE: See label example
Organic
USDA organic regulations describe the specific standards required for a company to
use the word “organic” or the USDA organic seal on food, feed, or fiber products. See
https://www.ams.usda.gov/services/organic-certification and/or
https://www.ams.usda.gov/rules-regulation/organic/labeling.
The USDA organic regulations are administered by AMS’s National Organic Program
(NOP) and described at 7 CFR Part 205. For animal products to be labeled as organic,
livestock producers must be certified organic and any operations that subsequently
handle the organic product must be certified organic (e.g., slaughter plants, meat

16
packing facilities). Organic operations are inspected annually by USDA-accredited
certifying agents. The label bearing the claim needs to include the certifying entity’s
name, website address, and logo, when the organization has a logo.
1. FSIS accepts current organic certificates to substantiate certain animal raising
claims such as: “raised without antibiotics,” “no added hormones,” “vegetarian
diet,” “no animal-by-products,” “NON-GMO” and “humanely raised” with
company’s description and qualifier [“These are consistent with the USDA
organic regulations”].
2. As referenced previously in this document, organic claims, logos and/or websites
from purchased products cannot be carried over to other products unless the
company has the same certification.
3. If an establishment produces meat or poultry products that qualify for an organic
claim under the NOP, the establishment would not need to provide a written
description of the product tracing and segregation mechanism because these
activities are a condition of NOP certification.
NOTE: See label example
Requesting approval for the addition of new suppliers to the documentation for a
product with a previously approved label bearing animal raising claims
LPDS has procedures in place that allow for an establishment to add a new supplier to
the documentation of a previously approved label and not have to resubmit the label for
another sketch approval. For the purposes of this section, a supplier is a producer,
farmer, or even another establishment that provides animals or products to another
establishment to use in its products that bear the same animal raising claims. Examples
would include but not limited to suppliers added for an approved product label bearing
animal raising claims, meat or poultry cuts for specific breeds. Additional suppliers
must be approved by LPDS and upon approval the labeling record must be updated to
reflect the new supplier(s). To obtain approval, the producing establishment would need
to submit a signed and dated request to LPDS by email or letter that includes the
following:
1. The product name;
2. The producing establishment’s name, address, and establishment number; the
prior label approval number; and a copy of the previously approved label
application;
3. The specific claim(s) that will be used on the product label containing source
materials from the new supplier;
4. The new supplier’s name, address, and one copy of any labels with the same
claim(s) previously approved by LPDS associated with the supplier or
documentation to support why the claim(s) also applies to the new supplier; and
5. Finally, for claims certified by a third-party, include a copy of the current
certificate(s). Due to the fact that most certifications expire after one year, FSIS
will consider a certificate current based on a one-year time span unless the
certificate states otherwise.

17
Once all the documentation above is evaluated, LPDS will notify the applicant in writing
of its final determination. As mentioned above, this letter needs to be included as part of
the supporting documentation in the producing establishment’s official labeling record.
Label Example
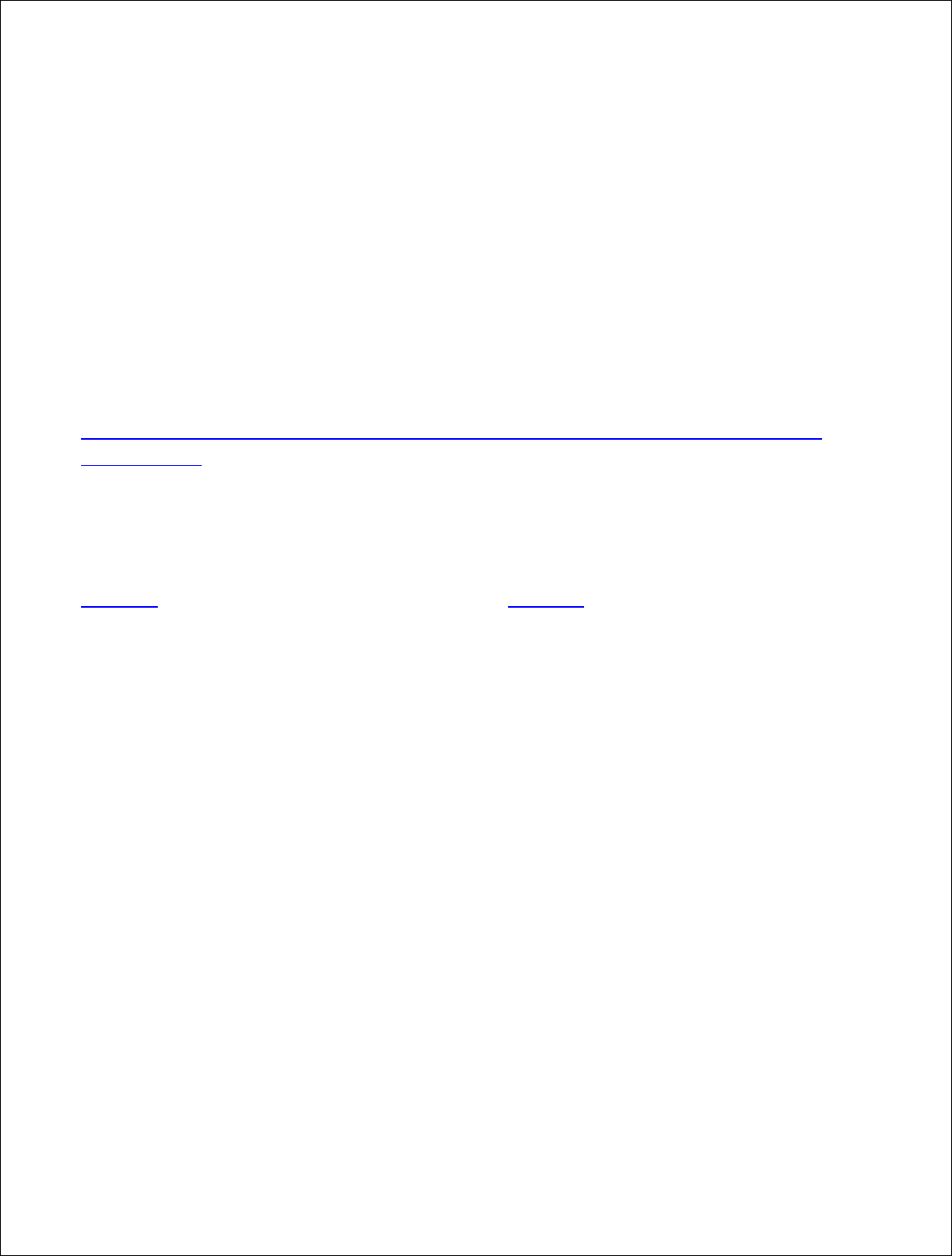
The above label contains the following types of claims:
- Breed (Angus);
- Diet (Grass-fed);
- Living/Raising/Raising Conditions (Free-Range);
- Raised Without Antibiotics – Livestock/Red Meat (No Added Antibiotics);
- Raised Without Added Hormones (No Added Hormones Administered);
- Source/Traceability (Source Verified and Traceable to TMB Ranch);
- Third-Party Certification (LPDS True 2 Earth); and
- Organic (USDA Organic)

